BamHI
Recognition and Cleavage Sites
5′ … G ↓G A T C C … 3′
3′ … C C T A G ↑G … 5′
Unit Definition
A unit of the enzyme is defined as the quantity needed to completely digest 1 µg of pLP-MCS001 DNA (Catalog No: SCV0001) within 1 hour at 37 °C in a 50 µL reaction volume using the assay buffer.
Features
• Optimized Buffer Recipe
• Improved Performance
• Reduced Star Activity
• UCut Buffer and storage buffer contains premixed BSA to enhance stability
Applications
• Molecular Cloning
• Restriction Site Mapping
• Restriction Verification
dam Methylation Sensitive: NO
dcm Methylation Sensitive: NO
CpG Methylation Sensitive: NO
Optimal Reaction Temperature: 37 °C
Restriction Enzyme Cut Site: G/GATCC
Heat Inactivation: No
Isoschizomers: N/A
Nuclease Contamination Control: Passed
Storage: -20 °C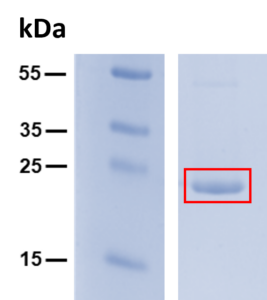
Figure 1: Purity of BamHI enzyme.
| Cat. No. | Component | Quantity | Storage | Concentration |
|---|---|---|---|---|
| MCE1001-S(4.000 unit) | BamHI | 1 x 0.4 ml | -20˚C | 20,000 U/ml |
| UCut Buffer | 1 x 1.5 ml | -20˚C | 6X | |
| Blue Gel Loading Dye | 1 x 1.5 ml | 25˚C | 10X | |
| MCE1001-L(16.000 unit) | BamHI | 4 x 0.4 ml | -20˚C | 20,000 U/ml |
| UCut Buffer | 2 x 1.5 ml | -20˚C | 6X | |
| Blue Gel Loading Dye | 2 x 1.5 ml | 25˚C | 10X |
| Reagent | Amount |
|---|---|
| Restriction Enzyme | 1 µl* |
| UCut Buffer | 5 µl |
| DNA | 1 µg** |
| Water (Ultra Pure) | X*** |
| Total Reaction Volume | 50 µl |
Incubate the enzyme reaction at 37 °C for 1 hour.
*When using multiple enzymes for digestion, the total enzyme volume should not exceed 5 µl in a 50 µl reaction.
**The DNA template should be of high purity, devoid of any residual impurities such as phenol or ethanol, which are known to inhibit restriction digestion reaction.
***Ultra-pure water is added to bring the total reaction volume to 50 µl. Problem 1: No digestion or incomplete digestion
Possible Causes:
• Incorrect enzyme concentration.
• Enzyme inactivation due to improper storage or handling.
• Inadequate incubation time or temperature.
• Inhibitory substances present in the DNA or buffer.
Solutions:
• Check enzyme amount: Ensure that the enzyme amount used is within the recommended range. For multiple enzymes, ensure the total enzyme volume does not exceed 5 µl in a 50 µl reaction.
• Verify enzyme activity: Ensure that the enzyme has been properly stored (usually at -20°C) and has not expired.
• Optimize incubation conditions: Incubate the reaction at the optimal temperature for the specific enzyme (usually 37°C). Some enzymes may require a longer incubation time than recommended (1–2 hours).
• Check the DNA purity: Ensure the DNA template is free of contaminants (such as proteins, RNA, or ethanol), which may inhibit the enzyme activity.
Problem 2: Partial digestion (only some fragments are digested)
Possible Causes:
• Incomplete mixing of reagents.
• Inappropriate reaction conditions.
• High DNA concentration leading to incomplete digestion.
Solutions:
• Ensure proper mixing: Gently mix the reaction components by pipetting or flicking the tube before incubation.
• Check DNA concentration: Avoid using excessive amounts of DNA (typically 1 µg per 50 µl reaction). High DNA concentration may inhibit enzyme action.
• Verify reaction conditions: Make sure the correct buffer. Use fresh enzyme if necessary.
Problem 3: Star activity (non-specific digestion)
Possible Causes:
• Incorrect enzyme storage or handling.
• Excessive enzyme concentration.
• Incorrect reaction conditions (e.g., too high or low temperature, too high salt concentration).
Solutions:
• Check enzyme storage: Ensure enzymes are stored at -20°C and have not been subjected to multiple freeze-thaw cycles.
• Adjust enzyme concentration: Reduce enzyme concentration if it exceeds recommended levels (typically no more than 5 µl in a 50 µl reaction).
• Optimize reaction conditions: Ensure the reaction is carried out in the correct buffer with the appropriate salt concentration. Avoid high salt or alcohol levels, which can cause star activity.
Problem 4: Uneven digestion (uneven bands in agarose gel)
Possible Causes:
• Uneven distribution of enzymes in the reaction mixture.
• DNA degradation or contamination.
Solutions:
• Mix thoroughly: Ensure the enzyme and buffer are well mixed with the DNA.
• Ensure DNA integrity: Check the quality of the DNA template.
• Degraded DNA may result in uneven digestion patterns. Use pure, high-quality DNA for digestion.
Problem 5: Excessive background (bands or smear not related to expected digestion pattern)
Possible Causes:
• Excessive enzyme or buffer contamination.
• Impure DNA template.
Solutions:
• Use fresh reagents: Check for contamination of enzymes, buffers, or water. Use freshly prepared or aliquoted reagents.
• Clean DNA template: Re-purify the DNA to remove any potential contaminants such as salts, proteins, or ethanol.
General Tips:
• Always use freshly thawed enzymes and buffers.
• Keep reactions on ice until ready to begin incubation.
• Check buffer compatibility with the enzymes and make sure the pH and salt concentrations are optimal.
• If troubleshooting persists, try performing the digestion with a smaller amount of DNA or a different batch of enzymes.
Note: Enzymes are supplied with Blue Gel Loading Dye (6X) and UCut Buffer (10X) suitable for single and double digestions.
Research Use Only
Related Additional Materials
| Product | Cat. No. | Quantity | Storage | Concentration |
|---|---|---|---|---|
| Blue Gel Loading Dye | MCR0001 | 1 x 1.5 ml | 25˚C | 6X |
| UCut Buffer | MCR0002 | 1 x 1.5 ml | -20˚C | 10X |